
Chromium (III) compounds are not classifiable as to their carcinogenicity to humans (Group 3). Ingested nitrate or nitrite under conditions that result in endogenous nitrosation is probably carcinogenic to humans (Group 2A). Nitrites, nitrates, and their metabolites are excreted in the urine. The major metabolic pathway for nitrate is conversion to nitrite, and then to ammonia. In vivo conversion of nitrates to nitrites can occur in the gastrointestional tract under the right conditions, significantly enhancing nitrates' toxic potency. Intake of some amount of nitrates and nitrites is a normal part of the nitrogen cycle in humans. Chromium is almost entirely excreted with the urine. Inside the cell, hexavalent chromium is reduced first to pentavalent chromium, then to trivalent chromium by many substances including ascorbate, glutathione, and nicotinamide adenine dinucleotide.

Hexavalent chromium's similarity to sulfate and chromate allow it to be transported into cells via sulfate transport mechanisms. Bone is also a major storage site and may contribute to long-term retention. ( 5, 13, 1, 6, 2, 3, 4)Ĭhromium is absorbed from oral, inhalation, or dermal exposure and distributes to nearly all tissues, with the highest concentrations found in kidney and liver. This elevation of methemoglobin levels is a condition known as methemoglobinemia, and is characterized by tissue hypoxia, as methemoglobin cannot bind oxygen. Nitrite causes the autocatalytic oxidation of oxyhemoglobin to hydrogen peroxide and methemoglobin. Nitrate's toxicity is a result of it's conversion to nitrite once in the body. Chromium may increase its own toxicity by modifying metal regulatory transcription factor 1, causing the inhibition of zinc-induced metallothionein transcription. It can also cause transcriptional repression by cross-linking histone deacetylase 1-DNA methyltransferase 1 complexes to CYP1A1 promoter chromatin, inhibiting histone modification.
It has been shown to induce carcinogenesis by overstimulating cellular regulatory pathways and increasing peroxide levels by activating certain mitogen-activated protein kinases. Trivalent chromium may also form complexes with peptides, proteins, and DNA, resulting in DNA-protein crosslinks, DNA strand breaks, DNA-DNA interstrand crosslinks, chromium-DNA adducts, chromosomal aberrations and alterations in cellular signaling pathways.
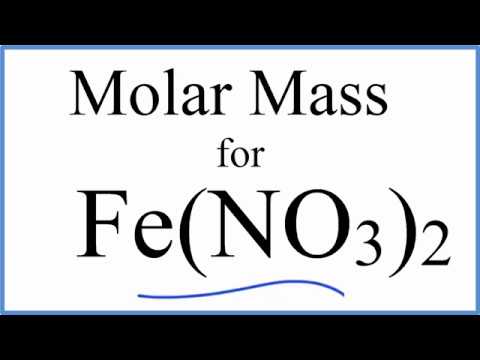

The anhydrous salt forms green crystals and is very soluble in water (in contrast to anhydrous chromium(III) chloride which dissolves very slowly except under special conditions). The chromium centers are bound to six aquo ligands, and the remaining volume of the solid is occupied by three nitrate anions and three molecules of water of crystallization. 3H 2O - betray a simple structure of this material.The relatively complicated formula - (NO 3) 3 It is common in academic laboratories for the synthesis of chromium coordination complexes. Chromium(III) nitrate compounds are of a limited commercial importance, finding some applications in the dyeing industry. Most common is the dark violet hygrosscopic solid. Except where otherwise noted, data are given for materials in their standard state (at 25 ☌, 100 kPa).Ĭhromium(III) nitrate describes several inorganic compounds consisting of chromium, nitrate and varying amounts of water.



 0 kommentar(er)
0 kommentar(er)
